

A good electrolyte must have low reactivity with other cell components, high ionic conductivity, low toxicity, a large window of electrochemical voltage stability (0-5V), and be thermally stable. In lithium-ion batteries, the electrolyte is typically a lithium salt dissolved in organic solvents. The choice of electrolyte in all batteries is critical for performance as well as safety. Depending on the type of battery, it can be a liquid or paste-like substance. The battery electrolyte is a solution inside batteries.

An electrolyte is a medium containing ions that are electrically conducting through the movement of those ions but not conducting electrons. Lithiated graphite has a unit cell with an HCP structure. New materials such as those based on Silicon and other elemental blends are being researched. One of the most common anode materials used today is lithiated graphite, Li xC 6, which is composed of graphite sheets intercalated with lithium. The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction. For the cathode, it is important to hold a large amount of lithium without significant change in structure, have a good chemical and electrochemical stability with electrolyte, be a good electrical conductor and diffuser of lithium ions, and be of low cost. In the case of lithium batteries, cathode materials are generally constructed from LiCoO 2 or LiMn 2O 4. The cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. However, most batteries have some common components, although their material composition may vary. Therefore, each battery has a different composition. The chemical and material composition of batteries determines their size, format, and overall performance.
#BATTERY CATHODE AA PORTABLE#
Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid, making it suitable for portable equipment.
/Batteries-58dd19023df78c5162a2b436.jpg)
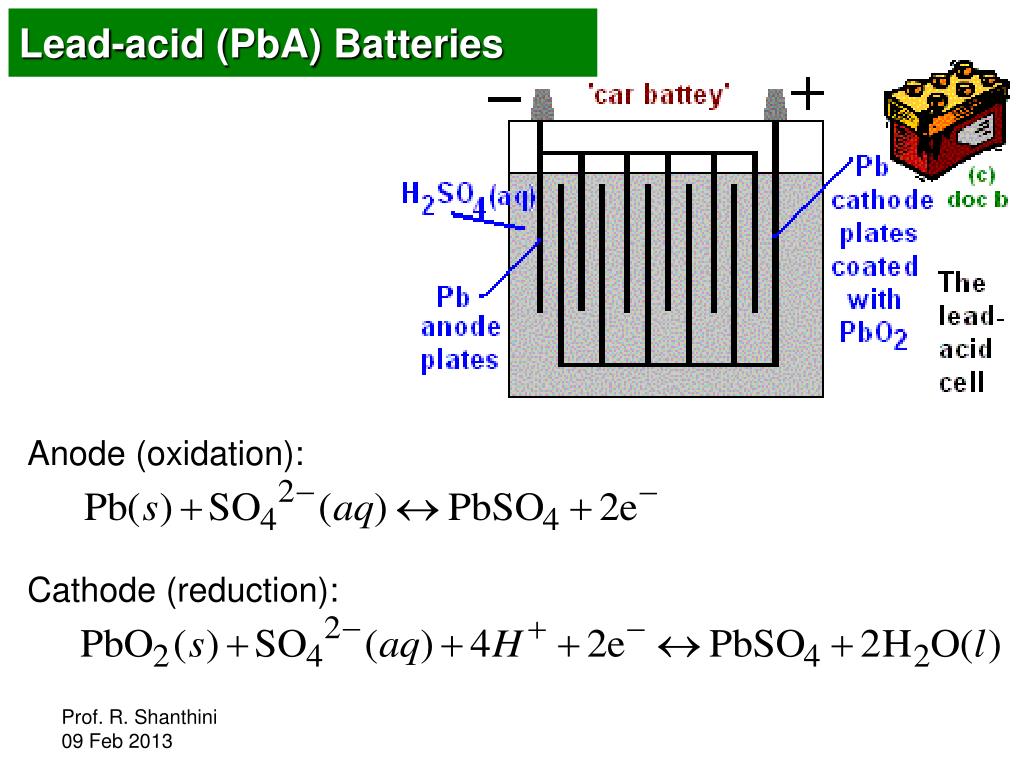
A dry cell uses a paste electrolyte with only enough moisture to allow current to flow.Wet cells were a precursor to dry cells and are commonly used as a learning tool for electrochemistry. Other names are flooded cell since the liquid covers all internal parts, or vented cell since gases produced during operation can escape to the air. A wet cell battery has a liquid electrolyte.Many types of electrochemical cells have been produced, with varying chemical processes and designs, including galvanic cells, electrolytic cells, fuel cells, flow cells, and voltaic piles. The most common ones are lead, nickel, zinc, and lithium, each of them with different outputs and specific for some different purposes depending on the requirements. Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.īatteries are made of an extensive range of materials resulting in different capabilities and behaviors in the functionality of the battery. Even though many types of batteries exist with different combinations of materials, all of them use the same principle of the oxidation-reduction reaction. The fundamental principle in an electrochemical cell is spontaneous redox reactions in two electrodes separated by an electrolyte, which is a substance that is ionic conductive and electrically insulated.Ĭhemical energy can be stored, for example, in Zn or Li, which are high-energy metals because they are not stabilized by d-electron bonding, unlike transition metals. A typical battery consists of one or more voltaic cells. This then provides a source of electromotive force to enable currents to flow in electric and electronic circuits. It converts stored chemical energy into electrical energy through an electrochemical process. An electric battery is essentially a source of DC electrical energy.


 0 kommentar(er)
0 kommentar(er)
